Discussion
Discussion
Vykat XR (diazoxide choline) for Prader-Willi syndrome
Last updated April 1, 2025, by Marta Figueiredo, PhD

What is Vykat XR for Prader-Willi syndrome?
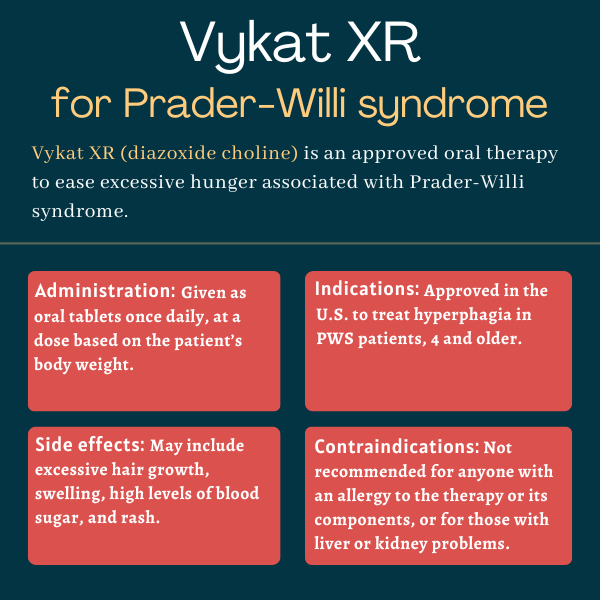
Vykat XR (diazoxide choline) is an oral therapy approved in the U.S. to reduce excessive hunger, or hyperphagia, in adults and children, 4 years and older, with Prader-Willi syndrome (PWS).
Designed to reduce the levels of appetite-stimulating proteins and hormones, Vykat XR became the first approved therapy to address hyperphagia in PWS patients. It is administered once-daily in the form of oral tablets.
Previously known as diazoxide choline controlled-release (DCCR), the therapy was developed and is marketed by Soleno Therapeutics, which is planning to file a regulatory application seeking the therapy’s approval in the European Union.
Therapy snapshot
| Brand name: | Vykat XR |
| Chemical name: | Diazoxide choline |
| Usage: | Used to reduce hyperphagia associated with PWS |
| Administration: | Oral tablets |
How does Vykat XR work?
Prader-Willi syndrome is a rare, complex genetic disorder caused by the loss of specific genes that control a wide range of bodily processes, including metabolism, growth, appetite, intellectual ability, and social behavior.
Symptoms of PWS include weak muscle tone and failure to thrive in infancy followed by the development during childhood of hyperphagia, which is marked by food obsession, aggressive food seeking, and lack of satiety.
As a result, patients are at risk for obesity and associated conditions such as heart and lung disease, high blood pressure, diabetes, and gastrointestinal complications. This adds to a range of other symptoms such as developmental delays, cognitive impairment, sleep problems, and behavioral challenges like temper outbursts.
Vykat XR is an extended-release formulation of diazoxide choline that acts on ATP-sensitive potassium (KATP) channels, which are gate-like proteins that open and close in response to the amount of blood sugar, or glucose, in the bloodstream.
By keeping KATP channels open in a region of the brain called the hypothalamus, the therapy reduces the release of neuropeptide Y and agouti-related peptide, which are appetite-stimulating proteins in the brain that are thought to drive hyperphagia.
Vykat XR also exerts some of its effects by acting on KATP channels in the pancreas, blocking the release of insulin, a hormone that helps move glucose from the blood into the body’s cells. With less insulin, more glucose remains in circulation, which may increase the feeling of satiety and reduce the buildup of fat. There is also evidence that keeping KATP channels open in fat tissue may be important for regulating body weight.
The specific formulation of diazoxide choline in Vykat XR allows the therapy to be dissolved in a controlled way for up to 24 hours, beginning in the stomach and then continuing in the bowel. This helps maintain a steady amount of the therapy in circulation with once-daily dosing.
By having an extended effect on KATP channels, daily Vykat XR is expected to help regulate appetite and lessen hyperphagia and associated aggressive behaviors, while limiting body fat buildup in people with PWS.
Who can take Vykat XR?
Vykat XR was approved by the U.S. Food and Drug Administration (FDA) in March 2025 to treat hyperphagia in adults and children, 4 and older, with PWS. The decision made it the first therapy to be approved for this burdensome PWS symptom.
A support program, called Soleno One, is available to help patients in the U.S. gain access to the therapy, as well as navigate insurance coverage and financial support to reduce out-of-pocket costs.
Who should not take Vykat XR?
Vykat XR is contraindicated, or not recommended, for anyone with a known allergy to diazoxide, any other ingredients in the medication, or thiazides, which is the class of molecules from which diazoxide is derived.
The therapy should not be given to patients with kidney or liver problems, or in combination with certain molecules and medications that are metabolized by the CYP1A2 enzyme. These include caffeine, the anti-inflammatory naproxen (sold as Aleve and others), the blood pressure-lowering propranolol (sold as Inderal and others), and certain antipsychotic therapies.
Vykat XR should be used with caution in people with compromised cardiac reserve, which refers to a reduced ability of the heart to increase its output in response to emotional or physical stress.
How is Vykat XR administered?
Vykat XR is available as extended-release tablets that are taken once daily. Tablets come in three dosage strengths:
- 25 mg white, capsule-shaped tablets carved with “S-25” on one side
- 75 mg white, round, convex-shaped tablets carved with “S-75” on one side
- 150 mg white, oval-shaped tablets carved with “S-150” on one side.
Vykat XR tablets can be taken with or without food, and should be swallowed whole and not split, crushed, or chewed.
Treatment with Vykat XR involves a six-week titration period, in which the starting dose is increased every two weeks to reach the target maintenance dose. The recommended dose is based on the patient’s body weight:
- those weighing 20 kg (about 44 lbs) to less than 30 kg (about 66 lbs) will receive a starting dose of 25 mg and a target maintenance dose of 100 mg (taken as one 75 mg tablet plus one 25 mg tablet)
- those weighing 30 kg to less than 40 kg (about 88 lbs) will start on a dose of 75 mg and reach a target dose of 150 mg (taken as one 150 mg tablet)
- those weighing 40 kg to less than 65 kg (about 143 lbs) will be given a starting dose of 75 mg and a target dose of 225 mg (taken as one 150 mg tablet plus one 75 mg tablet)
- those weighing 65 kg to less than 100 kg (220 lbs) will receive a starting dose of 150 mg and a target dose of 375 mg (taken as two 150 mg tablets plus one 75 mg tablet)
- those weighing 100 kg to less than 135 kg (about 298 lbs) will start on a dose of 150 mg and get to a target dose of 450 mg (taken as three 150 mg tablets)
- those weighing 135 kg or more will receive a starting dose of 150 mg and a target dose of 525 mg (taken as three 150 mg tablets plus one 75 mg tablet).
The maximum recommended daily dosage of Vykat XR is 5.8 mg/kg or 525 mg. However, starting and maintenance doses, as well as the maximum recommended daily dose, are all lower for patients taking medications that are strong CYP1A2 suppressors.
During the dose titration period, if patients develop clinically significant side effects, such as higher-than-normal levels of glucose or swelling, the dosage increase should be slower or aimed at a lower target dose. If these side effects occur during maintenance treatment, the dose might need to be reduced, or the treatment temporarily interrupted or discontinued altogether.
The medication can be discontinued without dose reduction. Patients restarting treatment after an interval of more than one week should undergo the process of dose titration again. For shorter treatment interruptions, Vykat XR can be resumed at the previous dose.
Vykat XR should not be substituted with diazoxide’s oral suspension formulation, as they have different pharmacological properties.
Vykat XR in Prader-Willi syndrome clinical trials
Soleno’s application to the FDA was supported mainly by data from a Phase 3 clinical trial called DESTINY PWS (NCT03440814) and its open-label extension C602 study (NCT03714373). The therapy’s efficacy was established during C602’s four-month withdrawal period, in which patients who were still receiving Vykat XR in the extension either continued treatment or were switched to a placebo.
DESTINY PWS
DESTINY PWS, also named C601, recruited 127 PWS patients, 4 and older, with moderate to severe hyperphagia at 29 sites in the U.S. and the U.K.
Participants were randomly assigned, in a 2:1 ratio, to receive either Vykat XR or a placebo, taken as oral tablets once daily for 13 weeks (about three months). Vykat XR dosage was increased to a target of 3.3 to 5.8 mg per kg of body weight within the first six weeks, with daily doses ranging from 100 to 450 mg.
The trial’s main goal was to assess how well Vykat XR worked, compared with the placebo, to ease hyperphagia. This was measured with the caregiver-reported Hyperphagia Questionnaire for Clinical Trials (HQ-CT), with scores ranging from 0 to 36, and higher scores reflecting more severe hyperphagia.
Secondary goals included changes in body fat, as well as caregiver and clinician evaluations of clinical progress. Exploratory goals included changes in other body composition parameters, certain hormones, and behavioral problems, as assessed with the caregiver-reported Prader-Willi Syndrome Profile (PWSP) Questionnaire and the Developmental Behavior Checklist version 2 parent edition (DBC2-P).
Top-line data showed Vykat XR treatment was associated with a greater reduction in hyperphagia compared with the placebo, but this difference did not reach statistical significance, failing to meet the trial’s main goal.
Still, the therapy resulted in a significantly greater HQ-CT score reduction, by more than twofold, relative to the placebo in a subgroup of patients with more severe hyperphagia at the beginning of the study (HQ-CT scores higher than 22).
Vykat XR-treated patients also experienced significant clinical improvements, as assessed by the investigators, and a significant drop in body fat compared with those given the placebo.
The therapy was also associated with a trend toward lessening in the anxiety, compulsivity, rigidity/irritability, and disordered thinking domains of the PWSP and a reduction in DBC2-P total scores, indicating less severe behaviors. However, these differences failed to reach statistical significance.
Further analyses suggested the COVID-19 pandemic greatly influenced the results on caregiver-reported measures, which are more subjective and susceptible to external influences. Indeed, an analysis covering pre-pandemic data showed significant reductions in hyperphagia and behavioral symptoms with Vykat XR, and the statistical significance was lost when the pandemic started.
C602
The majority of patients completing DESTINY PWS (90.6%) chose to enter its open-label extension study, called C602, where all would receive Vykat XR for up to three years.
Results showed one year of treatment significantly reduced hyperphagia and behavioral challenges, while significantly improving body composition (with greater lean body mass) and hormonal and metabolic profiles.
Data from interviews with caregivers of trial participants also supported the treatment’s beneficial effects for several behavioral symptoms.
Notably, all these trajectories were significantly different from what was seen over one year in untreated, matched patients participating in the PATH for PWS natural history study (NCT03718416).
While data from DESTINY PWS was initially submitted to the FDA, the regulatory agency called for more clinical data before an approval could be considered. This prompted an amendment to C602’s protocol to include a withdrawal period, where patients were randomly assigned to either continue on Vykat XR or switch to a placebo for four months. At that point, patients had been treated with Vykat XR in the main and/or extension trial for a mean of 3.3 years.
Results showed that those who switched from Vykat XR to the placebo experienced significantly greater hyperphagia worsening after four months compared with those who stayed on Vykat XR. Specifically, patients continuing Vykat XR treatment saw a 2.6-point increase in HQ-CT scores, while those on placebo saw a 7.6-point increase — a 5-point difference that was statistically significant.
The switch also resulted in significantly greater weight gains, while continuous Vykat XR treatment was associated with a trend toward greater clinician-rated improvements and behavioral symptom reductions.
C614
Eligible C602 study participants could choose to enter another open-label extension study, called C614 (NCT05701774), in which all are receiving Vykat XR treatment for up to five years.
Common side effects of Vykat XR
The most common side effects of Vykat XR reported in clinical trials include:
- excessive hair growth
- swelling
- higher-than-normal levels of glucose in the blood (hyperglycemia)
- rash.
Hyperglycemia
Due to its insulin-lowering effects, Vykat XR leads to an increase in glucose levels that can, in some cases, exceed the normal range. Hyperglycemia, including severe adverse events associated with diabetic ketoacidosis, have been reported in patients receiving the therapy.
Diabetic ketoacidosis is a potentially life-threatening condition in which insulin deficiency prevents cells from uptaking glucose (the body’s main energy source). The body will instead burn fat, and this process leads to the excessive accumulation of substances called ketones in the blood.
Therefore, blood levels of glucose and HbA1c — a marker of glucose levels in the last two to three months — should be measured before and regularly during Vykat XR treatment. Glucose monitoring should be done more frequently for patients with an increased risk of hyperglycemia, including those who are obese, have elevated HbA1c levels, or are also being treated with standard growth hormone or corticosteroids.
Patients developing signs or symptoms of hyperglycemia, such as excessive thirst, more frequent urination, or increased appetite with weight loss, should contact their healthcare provider. The same applies if patients experience any signs of diabetic ketoacidosis, including nausea, vomiting, abdominal pain, general feeling of being unwell, and shortness of breath.
When a patient has hyperglycemia, their glucose levels should be measured at least twice a week until levels drop to the normal range. Physicians may also consider measuring ketone levels in patients experiencing worsening hyperglycemia.
If patients are taking any anti-hyperglycemic medication, their glucose levels should continue to be monitored regularly for at least 10 weeks. Based on the severity of hyperglycemia, they may need to reduce their Vykat XR dosage, interrupt treatment, or stop it altogether to avoid progression to ketoacidosis.
Swelling
Vykat XR has antidiuretic properties, meaning that it reduces urination. As such, the therapy can cause edema, or accumulation of fluid that causes swelling.
In clinical trials, edema was reported in more than one-quarter of Vykat XR-treated patients. Some of these reactions were severe, including fluid overload in the lungs.
If patients experience trouble breathing or show other signs or symptoms of fluid overload, such as unusual swelling anywhere in the body, they should inform their healthcare provider, who may decrease the dose or temporarily stop Vykat XR treatment.
In theory, Vykat XR’s antidiuretic effects may contribute to heart failure in patients with compromised heart reserve. The therapy has not been tested in these patients, so caution is advised when considering Vykat XR for them.
Use in pregnancy and breastfeeding
Vykat XR has not been rigorously studied in women who are pregnant or breastfeeding. However, side effects reported in adults and available data from case reports suggest that exposure to the therapy during pregnancy may cause harm to the fetus.
In animal studies, Vykat XR exposure during pregnancy at doses higher than the therapy’s maximum recommended human dose were associated with some maternal and fetal toxicities.
Vykat XR is present in the breast milk of treated patients, but its potential effects on a breastfed infant or on milk production in nursing patients remain unknown. It may be advisable to monitor glucose levels in infants breastfed by patients on the therapy.
Patients who are pregnant or nursing, or plan to become pregnant or to breastfeed, should discuss this topic with their healthcare team, carefully weighing the potential risks and benefits of continuing or stopping Vykat XR treatment.
Prade-Willi Syndrome News is strictly a news and information website about the disease. It does not provide medical advice, diagnosis, or treatment. This content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
Recent Posts
- For rare disease families, February is a month of both love and awareness
- Muscle, bone signaling imbalances found in non-obese PWS children
- Most PWS patients meet nutrient targets but struggle with calorie control
- Adults with PWS need lifelong support in daily activities, study finds
- Celebrating a milestone in life with Prader-Willi syndrome
- New technique ‘wakes up’ silent genes in Prader-Willi in lab testing
- Probiotics may boost beneficial gut bacteria in Prader-Willi syndrome
- Avoiding meltdowns during the holidays with Prader-Willi syndrome
- PWS patients see weight loss, less hunger with setmelanotide in trial
- Eye-tracking test adapted to better measure hunger behaviors in PWS
Related content
-
 Discussion
Discussion